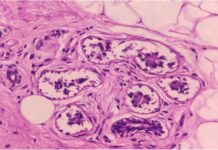
Lung cancer, a leading killer, has been hard to target with drugs. A team took a metabolic approach, looking at what lung tumor cells need to live and grow. When they removed these factors, tumor growth was almost completely suppressed in a mouse model. Their findings suggest that a combination of existing drugs (IGF-1 inhibitors and inhibitors of protein breakdown) could provide an alternative to chemotherapy in curbing this deadly cancer.
A new model of growth factors’ effect in lung cancer
Kalaany’s team created the new model by crossing two strains of mice: a well-established strain that models KRAS-driven lung cancer, and another strain that lacks insulin/IGF-1 signaling, previously developed by Morris White, PhD, of Boston Children’s to study diabetes. This second model deletes two key genes, known as Irs1 and Irs2, that encode so-called “adaptor” proteins that are necessary for insulin/IGF-1 signaling (a discovery made by White in the 1980s).
The new, cross-bred mice provide the best model yet for studying insulin/IGF-1 signaling in lung cancer. The researchers went on to show that when both Irs1 and Irs2 are deleted in the lungs, insulin/IGF-1 signaling is eliminated and lung tumors are strongly suppressed.
A metabolic approach to cancer
It turns out protein breakdown can also be inhibited with existing drugs, such as chloroquine, which inhibits autophagy (literally, “self-eating”) and is being used in several cancer drug trials, and bortezomib (Velcade), a so-called proteasome inhibitor that is used to treat multiple myeloma.
When Kalaany’s team injected human tumor cells lacking Irs1/2 into mice, tumors didn’t grow as well. When they added inhibitors of protein breakdown, growth was almost completely suppressed.